|
|

|
|
Healthmark Industries is proud to present the “Quick Comply” starter kit for your Endoscopy / GI departments.
Designed to comply with AAMI ST91, the Quick Comply Kit provides key elements for cleaning verification, safe storage, traceability, and labeling all in one convenient kit: 100 ChannelCheck™ (UCC-101) that come with 2 boxes of 50 test strips and 1 control per box capable of testing any lumened instrument for residual organic soils (i.e., blood, protein and carbohydrates); 20 Valvesafe™ (VS-002 BLU) single-use polypropylene cages that attach to endoscopes for storage of up to four endoscope valves and detachable parts, the unique container design has large openings that allow disinfectants to flow through effectively and permit adequate drying in a contaminant free environment; 1 Valvesafe™ Dispenser (VSH-022) made from ABS Plastic designed to store 20 single-use Valvesafe™ cages (VS-002 BLU); 100 Green HangTime Labels (403225 HTKG) that track your endoscope by day and month to indicate the scopes last reprocess date, which includes fillable spaces for the scope serial number and initials (labels are available in other colors). The Quick Comply Kit is available for individual purchase.
Part# Description Pkg Price
QCK-101 Quick Comply Kit EA $165.00
More information available upon request. Email US or Call: 713-640-5393
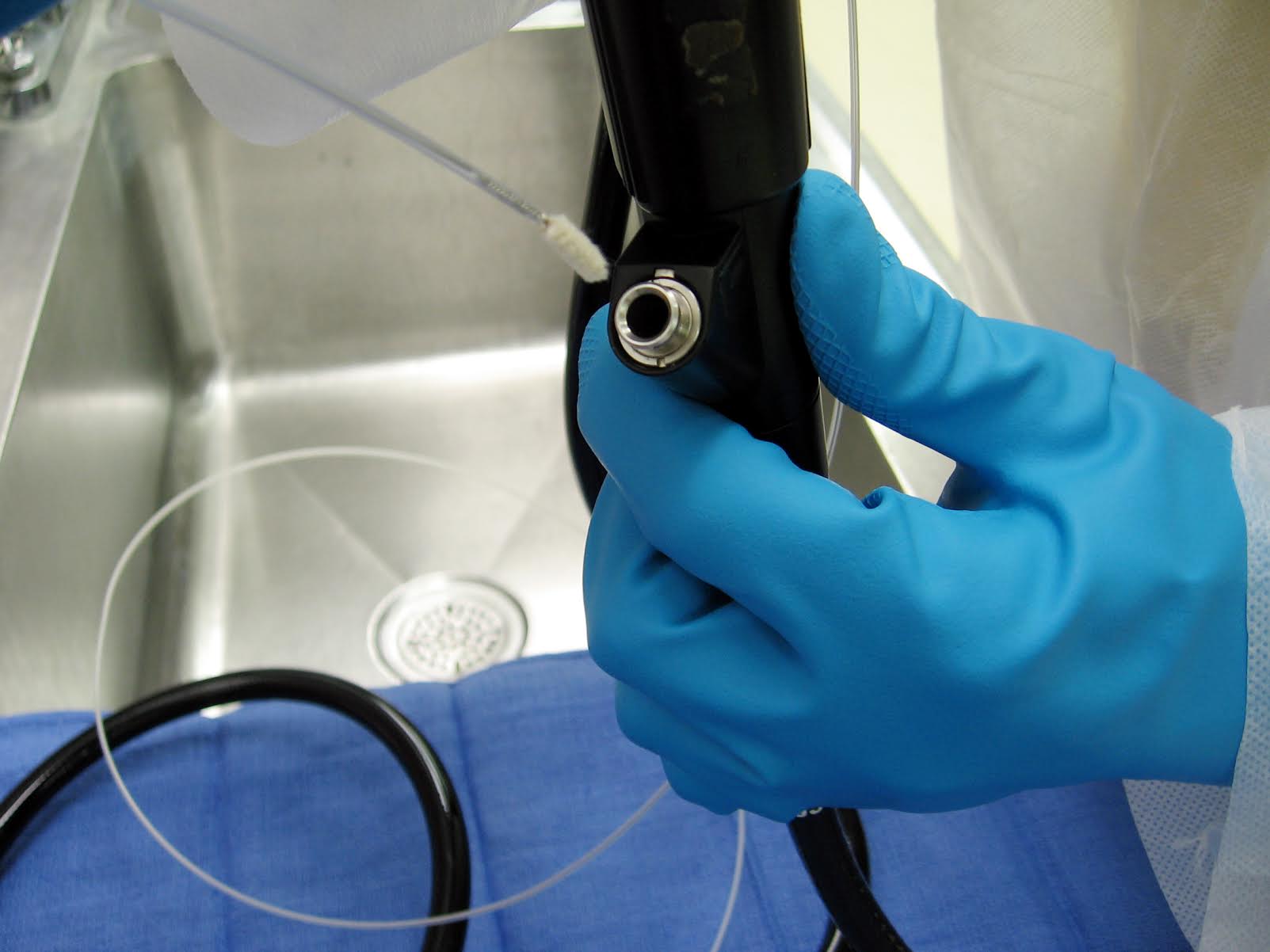
 According to a recent study, approximately 500,000 Bronchoscopes are performed nationwide each year. The FDA is taking a serious look at how meticulously these scopes are cleaned after each use. The team at Houston Hospital Services, Inc. in partnership with Healthmark Industries, offers solutions for some of the cleaning process failures outlined in this recent report. Below is an excerpt from that report. To read the report in its entirety, CLICK HERE
According to a recent study, approximately 500,000 Bronchoscopes are performed nationwide each year. The FDA is taking a serious look at how meticulously these scopes are cleaned after each use. The team at Houston Hospital Services, Inc. in partnership with Healthmark Industries, offers solutions for some of the cleaning process failures outlined in this recent report. Below is an excerpt from that report. To read the report in its entirety, CLICK HERE
Unfortunately, this isn’t something we are seeing LESS of in the news. However, we are confident steps are being taken at all levels to ensure patient safety and care. Healthmark Industries has an entire line of cleaning verification products that add very little time, money and effort to each procedure. Contact us today to learn more or to schedule your departmental in-service TODAY. (713) 640-5393 or team@hhstx.com
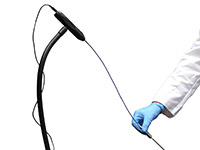
Did you know that your flexible scope cleaning station could be equipped with a flexible inspection scope verification tool like this one? It’s a small price to pay to ensure that all the ‘nooks and crannies’ are being scrubbed and cleaned of bio-burden. No human eye can possibly detect all the possible places bio-burden may be hiding. Why put your patient and your hospital at risk? Take the extra step and get a better view of your scopes once they have been “cleaned”. Need further verification? Healthmark Industries is the leader is cleaning verification tools for these scopes. With EndoCheck™, ChannelCheck™, Pro-Check™ and HemoCheck™. Click here to learn more or call Houston Hospital Services, Inc at 713-640-5393 for more information!

If you work in or around the hospital heathcare industry, you know the hot button today is the verification of flexible scope cleanliness. Houston Hospital Services is developing a service for hospitals in the Texas Medical Center and surround hospital systems in Houston using ProFormance™ Cleaning Verification – EndoCheck from Healthmark Industries.
With patient safety and hospital procedures in mind, HHS intends to develop an easy to read / easy to implement verification check for flexible scopes without adding a ton of additional work to the SPD or Endoscopy departments.
With that in mind, can you safely answer the question “Are your Flexible scopes ready for the next step”? All scopes should be verified “clean” from Protein, Blood and Carbohydrates before placing the scope in your AER. Houston Hospital Services, Inc. along with Healthmark Industries has created a “program” that is simple and easy to follow. It adds very little time, effort and money to each procedure keeping patient safety in mind. Let our team provide you with tools to check and verify the cleanliness of your scopes. (713) 640-5393