- Germs are everywhere.
- Infections cause great harm.
- Copper kills infectious bacteria.
Copper Kills MRSA



Houston Hospital Services is the proud distributor for Logiquip Storage Solutions. At the IAHCSMM Conference and Expo, Logiquip launched a newly designed Endoscope drying cabinet. Logiquip is proud to offer a full line of endoscope cabinets, the Endo Dri-Stor series. Fabricated using high-quality stainless steel and available with a broad range of options including channel air purge. Designed to stylishly protect and manage scopes.
FEATURES:
• All welded type 304 stainless steel fabrication.
• Every surface is non-porous and can withstand hospital-grade disinfectants.
• Hinged glass doors with stainless steel 1-1 /2″ tubular frame and piano hinge.
• Innovative side-compartment design allows easy access for to interior components and cabinet modularity. Two storage cabinets can attach to one component cabinet, which provides significant space and cost savings
• The modular design allows for options to be added later, expanding with your needs.
• Removable bottom stainless steel drip tray allows access to adjustable leveling feet and also to clean the floor beneath the cabinet.
• LED Lighting
• More ventilation options than any other cabinet made in North America. Choose from: – Ambient without filter – Power vent with HEPA Filter – Channel Air Purge with HEPA Filter – Power vent with HEPA Filter and Individual Channel Purge
• Premium HEPA filter: removes 99:97% of particulate 0.3 micron in size
The Industry Demands REAL Channel Purge Drying Cabinets!
Drying is a critical element in reprocessing … Endoscopes must be stored in an area that is clean, well ventilated and dust-free in order to keep the endoscopes dry and free of microbial contamination …. Drying cabinets are designed to control air quality and humidity, and access to endoscopes” SGNA, Standards of Infection Prevention in Reprocessing of Flexible Gastrointestinal Endoscopes, 2015
For more information or to contact us for a quote, please call 713-640-5393 or email team@hhstx.com
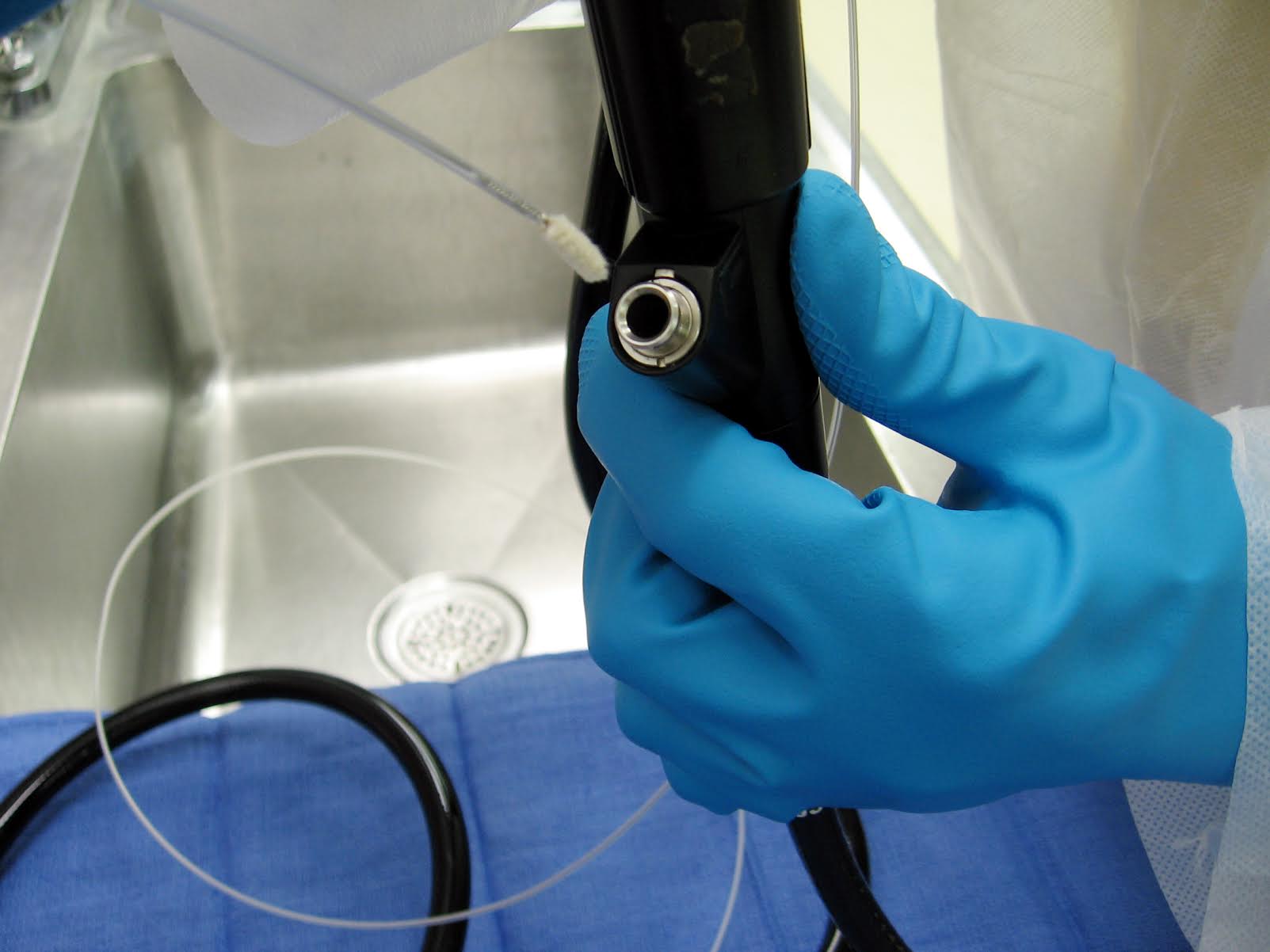
 According to a recent study, approximately 500,000 Bronchoscopes are performed nationwide each year. The FDA is taking a serious look at how meticulously these scopes are cleaned after each use. The team at Houston Hospital Services, Inc. in partnership with Healthmark Industries, offers solutions for some of the cleaning process failures outlined in this recent report. Below is an excerpt from that report. To read the report in its entirety, CLICK HERE
According to a recent study, approximately 500,000 Bronchoscopes are performed nationwide each year. The FDA is taking a serious look at how meticulously these scopes are cleaned after each use. The team at Houston Hospital Services, Inc. in partnership with Healthmark Industries, offers solutions for some of the cleaning process failures outlined in this recent report. Below is an excerpt from that report. To read the report in its entirety, CLICK HERE
Unfortunately, this isn’t something we are seeing LESS of in the news. However, we are confident steps are being taken at all levels to ensure patient safety and care. Healthmark Industries has an entire line of cleaning verification products that add very little time, money and effort to each procedure. Contact us today to learn more or to schedule your departmental in-service TODAY. (713) 640-5393 or team@hhstx.com

On February 27, 2016, Houston Hospital Services, Inc. represents Healthmark Industries at the local Houston SGNA Annual Spring Conference and Tradeshow! Located at the Doubletree by Hilton Hotel Greenway from 6:30am until 5:00pm. Join us to see what’s new from Healthmark Industries and how Houston Hospital Services can help your Gastroenterology departments with the latest tools in reprocessing and cleaning verification. Anyone centered around the Gastroenterology or Endoscopy departments in the Texas Medical Center and surrounding hospital systems are encouraged to attend.

If you work in or around the hospital heathcare industry, you know the hot button today is the verification of flexible scope cleanliness. Houston Hospital Services is developing a service for hospitals in the Texas Medical Center and surround hospital systems in Houston using ProFormance™ Cleaning Verification – EndoCheck from Healthmark Industries.
With patient safety and hospital procedures in mind, HHS intends to develop an easy to read / easy to implement verification check for flexible scopes without adding a ton of additional work to the SPD or Endoscopy departments.
With that in mind, can you safely answer the question “Are your Flexible scopes ready for the next step”? All scopes should be verified “clean” from Protein, Blood and Carbohydrates before placing the scope in your AER. Houston Hospital Services, Inc. along with Healthmark Industries has created a “program” that is simple and easy to follow. It adds very little time, effort and money to each procedure keeping patient safety in mind. Let our team provide you with tools to check and verify the cleanliness of your scopes. (713) 640-5393

Houston Hospital Services, Inc. features the new FlashPak® sterilization system from Symmetry Surgical. See below to view the demo and learn how easy it is to use this system. If you need to schedule an in-service demonstration, please call (713) 640-5393. If you need to order some for your hospital system, you can either contact us or Symmetry Surgical directly.

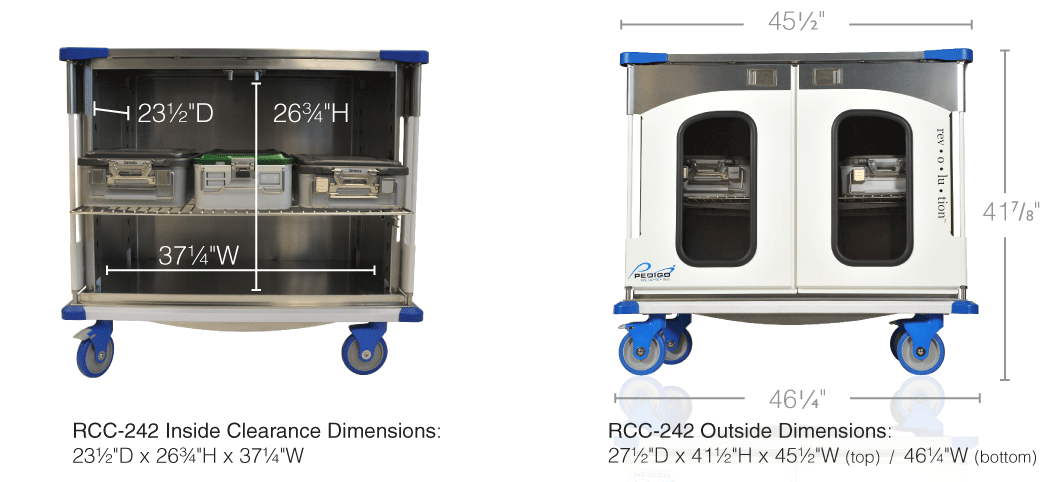
After a recent educational visit from the regional Pedigo USA representative, the Houston Hospital Services team learned about the NEW rev-o-lu-tion™ case carts. During the visit we discussed the differences in having “windows” vs solid stainless steel doors. The rev-o-lu-tion™ case carts features clear impact resistant windowed doors and a sleek new design. The aluminum doors are powder coated and can be customized with your hospitals colors or department colors. Although Pedigo USA continues to manufacture their USA made stainless steel case carts, the new rev-o-lu-tion™ case carts are a great improvement for any hospital system. To learn more CLICK HERE



This week, Houston Hospital Services, Inc. hosted the South Region VP of Pedigo. Although a short trip, he gave us insight as to why Pedigo is the clear choice for any hospitals stretcher needs. The 7500 Trauma Stretcher is the only USA made stretcher featuring a 6th wheel technology. The middle two wheels are spring action and roll evenly on all types of surfaces. Another key feature, exclusive to this line, is the new CuVerro® antimicrobial copper alloy handrails. Copper is a natural antimicrobial surface. Think about it. The handrails of stretchers are a high touch area. Wouldn’t you prefer to have this technology to aide in the sterility of your hospital?
Read More about this awesome new line and let us know your thoughts!