According to a recent study, approximately 500,000 Bronchoscopes are performed nationwide each year. The FDA is taking a serious look at how meticulously these scopes are cleaned after each use. The team at Houston Hospital Services, Inc. in partnership with Healthmark Industries, offers solutions for some of the cleaning process failures outlined in this recent report. Below is an excerpt from that report. To read the report in its entirety, CLICK HERE
According to a recent study, approximately 500,000 Bronchoscopes are performed nationwide each year. The FDA is taking a serious look at how meticulously these scopes are cleaned after each use. The team at Houston Hospital Services, Inc. in partnership with Healthmark Industries, offers solutions for some of the cleaning process failures outlined in this recent report. Below is an excerpt from that report. To read the report in its entirety, CLICK HERE
- Failure to meticulously follow manufacturer instructions for reprocessing, including:
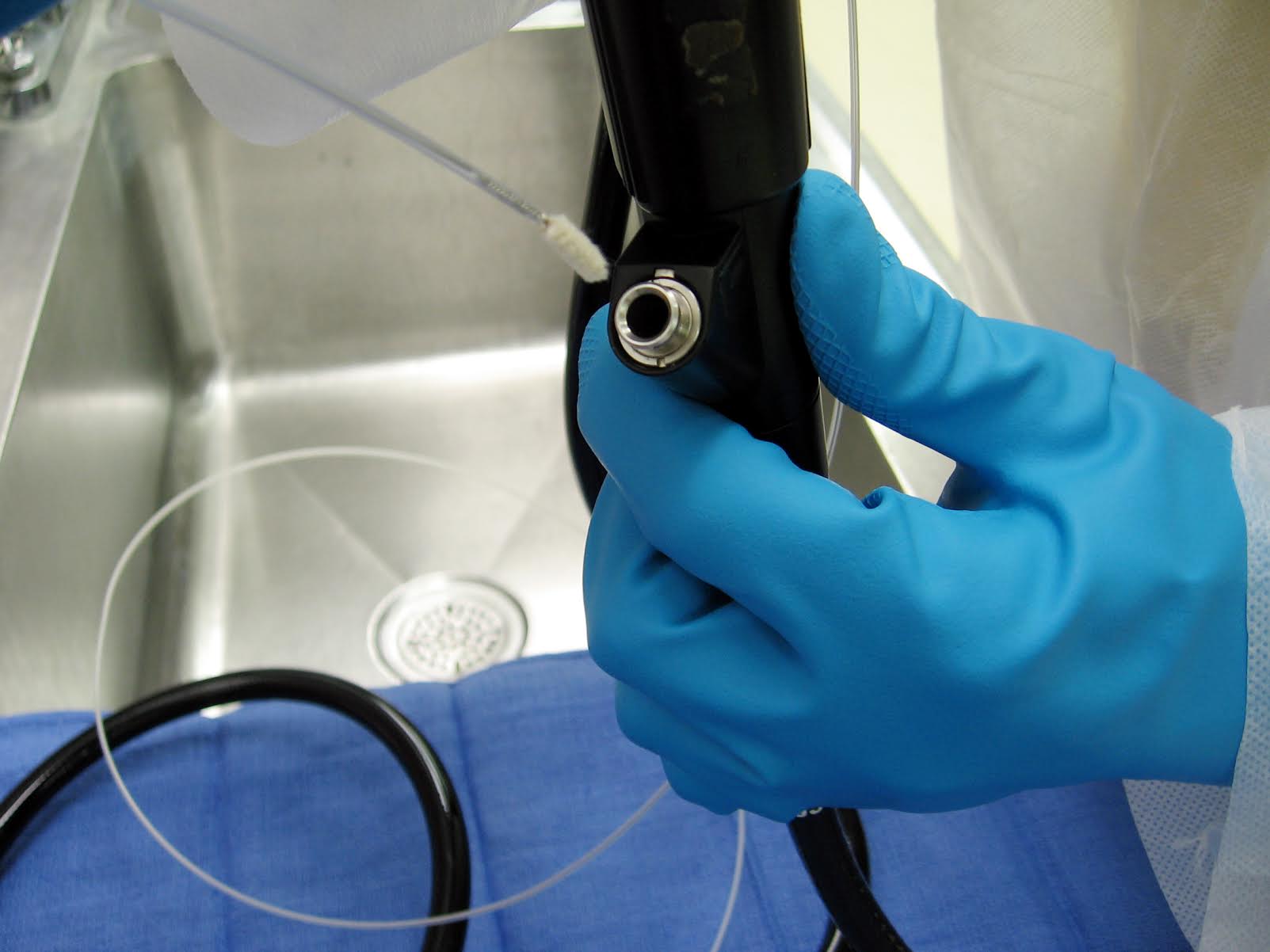
- Lack of pre-cleaning at point of use. Pre-cleaning typically includes surface wiping and channel flushing to prevent drying of blood, tissue and other biological debris;
- Failure to perform thorough manual cleaning before high-level disinfection (HLD) or sterilization;
- Failure to flush or brush channels;
- Use of expired detergent or high-level disinfectant;
- Insufficient flushing, rinsing and/or drying after HLD.
- Continued use of devices despite integrity, maintenance and mechanical issues, including:
- Persistent device channel kinks or bends;
- Channel wall scratches, divots, or crevices;
- Holes, cracks, or other imperfections in the distal end;
- Use of repaired or refurbished devices using out-of-specification parts;
- Use of devices despite residual material in the instrument or suction channels.
Unfortunately, this isn’t something we are seeing LESS of in the news. However, we are confident steps are being taken at all levels to ensure patient safety and care. Healthmark Industries has an entire line of cleaning verification products that add very little time, money and effort to each procedure. Contact us today to learn more or to schedule your departmental in-service TODAY. (713) 640-5393 or team@hhstx.com